Published online Feb 27, 2024. doi: 10.4254/wjh.v16.i2.126
Peer-review started: November 30, 2023
First decision: December 23, 2023
Revised: December 31, 2023
Accepted: January 29, 2024
Article in press: January 29, 2024
Published online: February 27, 2024
Processing time: 89 Days and 6.5 Hours
This editorial describes the contemporary concepts of prevention and management of gastroesophageal variceal bleeding in liver cirrhosis (LC) patients according to the current guidelines. Gastroesophageal variceal bleeding is the most dangerous complication of portal hypertension in LC patients. Risk stratification and determination of an individual approach to the choice of therapeutic measures aimed at their prevention and management has emerged as one of the top concerns in modern hepatology. According to the current guidelines, in the absence of clinically significant portal hypertension, etiological and non-etiological therapies of LC is advisable for the primary preventing gastroesophageal variceal bleeding, whereas its presence serves as an indication for the administration of non-selective β-blockers, among which carvedilol is the drug of choice. Non-selective β-blockers, as well as endoscopic variceal ligation and transjugular intrahepatic portosystemic shunt can be used to prevent recurrence of gastroesophageal variceal bleeding. Pharmacotherapy with vasoactive drugs (terlipressin, somatostatin, octreotide), endoscopic variceal ligation, endovascular techniques and transjugular intrahepatic portosystemic shunt are recommended for the treatment of acute gastroesophageal variceal bleeding. Objective and accurate risk stratification of gastroesophageal variceal bleeding will allow developing individual strategies for their prevention and management, avoiding the first and further decompensation in LC, which will improve the prognosis and survival of patients suffering from it.
Core Tip: Given that gastroesophageal variceal bleeding is the most dangerous complication of portal hypertension, objective and accurate risk stratification will allow developing individual strategies for their prevention and management, avoiding the first and further decompensation in liver cirrhosis, which will improve the prognosis and survival of patients suffering from it. This editorial describes the contemporary concepts of prevention and management of gastroesophageal variceal bleeding in liver cirrhosis patients according to the current guidelines.
- Citation: Garbuzenko DV. Contemporary concepts of prevention and management of gastroesophageal variceal bleeding in liver cirrhosis patients. World J Hepatol 2024; 16(2): 126-134
- URL: https://www.wjgnet.com/1948-5182/full/v16/i2/126.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i2.126
Liver cirrhosis (LC) is the final stage of many chronic liver diseases and has long been considered a static, irreversible pathological process. However, research in recent years has refuted this well-established notion. LC is now considered as a dynamic, potentially reversible disorder, where there are a compensated stage (without or with clinically significant portal hypertension (CSPH), which is characterized by hepatic venous pressure gradient (HVPG) values ≥ 10 mmHg and gastroesophageal varices (GEV) forming) and a decompensated stage, often accompanied by fatal complications associated with portal hypertension (PH) and/or liver failure[1]. Hence, LC decompensation is the most important stratification variable of a bad prognosis[2]. After the first decompensation, a progressive growth in portal pressure increases a likelihood of further decompensation, a resistance to treatment, a risk of death, and the need for liver transplantation[3].
Based on the modern concept of the natural history of LC, at the Baveno VI consensus workshop, held in 2015, recognized the use of “advanced chronic liver disease” as a term equivalent to LC to refer to cases of chronic liver disease with a risk of complications[4]. Since LC is an exclusively histological term, the approval of the new concept expanded the spectrum of the clinical course of the disease, allowing non-invasive methods to be used for its diagnosis and staging. Now LC patients can be stratified by a risk of complications, and therapeutic approaches are individualized. In 2021, at the Baveno VII consensus workshop, the main provisions adopted at the Baveno VI workshop were approved, and given the latest achievements, practical recommendations for personalized care for PH were developed[5].
This editorial describes the contemporary concepts of prevention and management of gastroesophageal variceal bleeding in LC patients according to the current guidelines.
The development of CSPH in compensated LC patients is an important prognostic factor, since it leads to an increased risk of first decompensation, one of the clinical manifestations of which is gastroesophageal variceal bleeding[1]. The gold standard of its diagnosis is HVPG measurement[6]. The normal range of HVPG is 1-5 mmHg, whereas a values of ≥ 10 mmHg indicates the presence of CSPH[7]. It should be recognized that until now, HVPG measurement is possible only in specialized centers. In addition, the procedure invasiveness and the need for repeated measurements increases a risk of possible complications and raises costs.
These limitations have contributed to the development of non-invasive methods for assessment of advanced chronic liver disease. One of them is the liver stiffness measurement by transient elastography. This is a fast, simple to perform, and are well tolerated procedure by patients with immediately available results[8]. According to the current guidelines, liver stiffness values by transient elastography of less than 10 kPa in the absence of other known clinical/imaging signs excludes LC, a values between 10 and 15 kPa suggest of it, and a values of more than 15 kPa indicates the presence of LC with a high probability[9]. At the Baveno VII consensus workshop, criteria were established for the exclusion or identification of CSPH by liver stiffness values that, combined with platelet count. According to them, liver stiffness values by transient elastography of more than 25 kPa indicates the presence of CSPH, whereas with liver stiffness values of less than 15 kPa and a normal platelet count, it is unlikely. LC patients with liver stiffness values between 20 and 25 kPa and platelet count less than 150 × 109/L or with liver stiffness values between 15 and 20 kPa and platelet count less than 110 × 109/L have a risk of developing CSPH with a probability of about 60%. They need additional screening[5]. For example, esophagogastroduodenoscopy (EGDS) has traditionally been used to detect GEV, the degree of dilation of which correlates with HVPG and, accordingly, with a risk of bleeding[10].
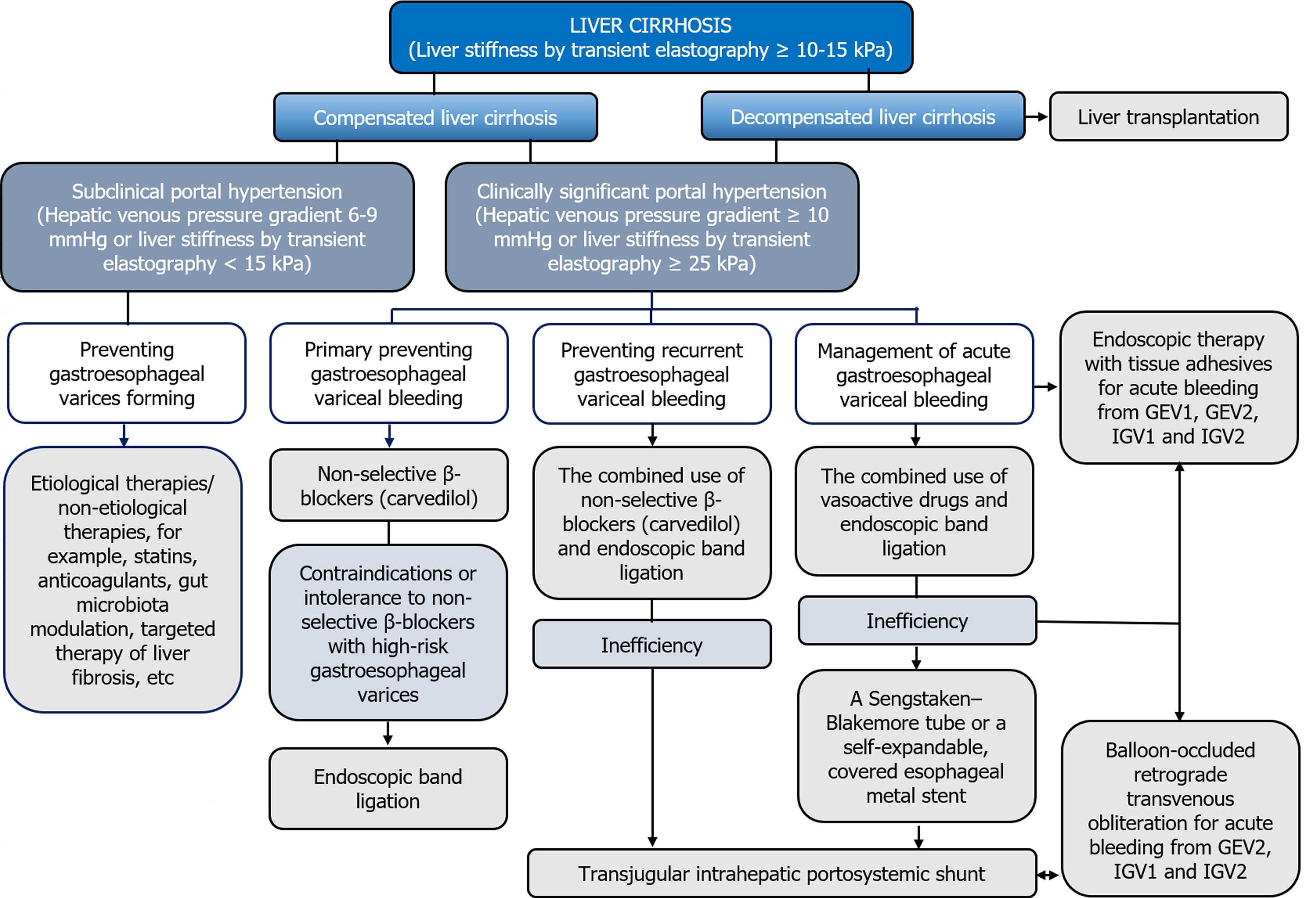
Therapeutic measures in compensated LC patients with or without CSPH, should be aimed at preventing first decompensation, in particular, primary preventing gastroesophageal variceal bleeding (Figure 1). In the absence of hyperdynamic circulatory state, etiological and non-etiological therapies of LC may be beneficial for preventing GEV forming[11]. Indeed, it has been found that alcohol abstinence positively affects the prognosis in alcohol-related LC, including patients with CSPH[12], and a sustained virologic response in LC associated with chronic HBV and HCV infection improves liver morphology and reduces HVPG[13]. The use of statins[14], anticoagulants[15], gut microbiota modulation[16] and targeted therapy of liver fibrosis[17] seems promising as a non-etiological treatment.
The drugs of choice for the primary preventing gastroesophageal variceal bleeding are non-selective β-blockers (NSBB), which is associated with their positive effect on the hyperdynamic circulatory state in CSPH. They can both reduce heart rate and cardiac output by blocking β1-adrenergic receptors, and decrease portal inflow as a result of splanchnic vasoconstriction caused by an endogenous α-adrenergic effect against the background of the blocking vasodilating β2-adrenergic receptors. In addition, NSBBs suppress the small intestinal bacterial overgrowth and prevent bacterial translocation contributing to systemic inflammation characteristic of LC, by accelerating orocecal transit[18].
Liver stiffness values of more than 25 kPa in compensated LC patients, which indicates the presence of CSPH, may be a criterion for prescribing NSBBs[19]. In the PREDESCI trial, the administration of NSBBs (propranolol and carvedilol) reduced HVPG and improved hyperdynamic circulatory state, which contributed to lowering the risk of first decompensation in compensated LC patients with CSPH[20]. The results of the PREDESCI trial were confirmed by quantifying the benefits of NSBBs in preventing LC decompensation[21]. A meta-analysis of 15 studies has shown that a reduction in portal pressure by NSBBs contribute to lowering the risk of complications, death or liver transplantation in LC patients[22]. Thus, the indication for prescribing NSBBs in compensated LC patients is a CSPH in the presence of GEV[5].
The aim of PH pharmacotherapy with NSBBs should be HVPG reduction to less than 12 mmHg or 20% of the baseline, without allowing significant arterial hypotension and other adverse effects. However, since HVPG measurement is not widely available, and a decrease in heart rate does not correlate with HVPG reduction, the dose of NSBBs is adjusted to the maximum tolerated doses[23]. According to the current guidelines, NSBBs should be prescribed at a dose that decrease heart rate at rest by 25% or up to 55 beats per minute at the initial bradycardia. Daily doses of propranolol can vary from 20 mg orally (initial) to 320 mg (maximum) and should be individually determined according to clinical response. The dose of carvedilol should be titrated from the initial daily dose of 6.25 mg. The maximum dose is 25 mg/d[24]. In some systematic reviews and meta-analyses, it has been shown that correctly determined therapeutic dosages of carvedilol more significantly reduce HVPG compared to propranolol, making it more effective in preventing gastroesophageal variceal bleeding in LC patients[25,26]. In the majority of responders to carvedilol therapy, the HVPG-response is maintained over a long period, which improves the clinical outcome[27]. As a consequence, at the Baveno VII consensus workshop, carvedilol was recommended as the drug of choice for preventing first decompensation in compensated LC patients with CSPH[5].
In compensated LC patients after the start of NSBBs therapy, there is no need to monitor the presence and dynamics of GEV during follow-up due to the lack of influence of the results of EGDS on therapeutic tactics. An exception may be in the case of a decision to withdrawal of NSBBs with the effectiveness of etiological treatment. In particular, their withdrawal is possible in patients who, 1-2 years after the elimination of the etiological factor, had a complete eradication of GEV and, according to transient elastography or HVPG, there are no signs of CSPH[28].
Endoscopic band ligation (EBL) is recommended in compensated LC patients with contraindications or intolerance to NSBBs with high-risk GEV for preventing first bleeding[5].
Further LC decompensation is an unfavorable prognostic stage associated with an even higher mortality rate than during the first decompensation, therefore, patients with it are candidates for liver transplantation. Further LC decompensation is characterized by recurrent gastroesophageal variceal bleeding, refractory ascites (requires > 3 large volume paracentesis within 1 year), recurrent encephalopathy, the development of spontaneous bacterial peritonitis, hepatorenal syndrome/acute kidney injury, as well as jaundice[5].
The combined use of NSBBs and EBL is the treatment of choice for secondary prophylaxis of gastroesophageal variceal bleeding[5]. This approach proved to be more effective than the use of each technique separately, both in preventing recurrent bleeding[29] and in improving survival[30]. With regard to the isolated use of NSBBs in secondary prophylaxis of gastroesophageal variceal bleeding, a recent systematic review showed the advantages of carvedilol over propranolol, which was accompanied by lower rates of recurrent bleeding, liver-related death, and further nonbleeding decom
In recent years, the issue of the expediency of prescribing NSBBs to decompensated LC patients with ascites has been discussed due to their ability to reduce increased cardiac output, which is a compensatory reaction to hypovolemia to maintain systemic and renal perfusion[32]. In this regard, at the Baveno VII consensus workshop, it was recommended that in decompensated LC patients with ascites, in case of persistently low blood pressure (systolic blood pressure < 90 mmHg or mean arterial pressure < 65 mmHg) and/or the presence of hepatorenal syndrome/acute kidney injury, the dose of NSBBs should be reduced or they should be completely canceled. Once blood pressure returns to baseline and/or after eliminating signs of hepatorenal syndrome/acute kidney injury, NSBBs can be re-initiated or re-titrated initially at a dose lower than when discontinuation. If patients remain intolerant to NSBBs, EBL is recommended to prevent gastroesophageal variceal bleeding[5].
Transjugular intrahepatic portosystemic shunt (TIPS) is the method of choice for preventing recurrent gastroesophageal variceal bleeding with the ineffectiveness of the combined use of NSBBs and EBL taking into account rebleeding severity and other complications of PH, with careful patient selection to minimize hepatic encephalopathy[33]. Compared to the combined use of NSBBs and EBL, polytetrafluoroethylene (PTFE)-covered TIPS have a significant benefit of preventing gastroesophageal variceal rebleeding[34], decrease the threat of further decompensation[35], and postoperative reduction of HVPG below 12 mmHg can contribute to recompensation[36].
At the Baveno VII consensus workshop, a number of changes and additions were made to the previously adopted recommendations for the management of LC patients with acute gastroesophageal variceal bleeding[5], although the general principles remained the same. If possible, all LC patients with acute gastroesophageal variceal bleeding should be hospitalized in the intensive care unit for resuscitation measures aimed at preserving tissue perfusion. It is important to quickly begin restoration of circulating blood volume to ensure and maintain hemodynamic stability. The threshold for red blood cell transfusion should be a hemoglobin level of 7-8 g/dL, taking into account factors such as cardiovascular diseases, age, hemodynamic status and the presence of ongoing bleeding. If there is a suspicion of acute gastroesophageal variceal bleeding, as early as possible, ideally before the EGDS, vasoactive drugs should be administered: terlipressin (under the control of serum sodium levels), somatostatin, octreotide for at least 5 d[37].
Terlipressin is usually administered 2 mg IV immediately, then 1-2 mg every 4-6 h until hemostasis achieved, or for 3 to 5 d. Somatostatin is administered 250 mg bolus IV initially, followed by 250 mg/h IV infusion for 3 to 5 d. Octreotide is administered 50 mcg bolus IV initially, followed by 50 mcg/h IV infusion until hemostasis achieved or for 3 to 5 d[38]. In a systematic review and meta-analysis, vasoactive drugs had similar indicators of mortality risk, control of acute gastroesophageal variceal bleeding, its early and late recurrence, need for transfusion of red blood cells and hospitalization duration. However, the use of terlipressin was accompanied by a higher risk of adverse events[39]. At the same time, the administration of proton pump inhibitors started before EGDS, after the diagnosis of acute gastroesophageal variceal bleeding in the absence of strict indications, should be stopped immediately, since their use in LC patients increases the likelihood of spontaneous bacterial peritonitis and other infectious complications[40].
Given the risk of bacterial infection primarily in decompensated LC patients with acute gastroesophageal variceal bleeding, antibiotic prophylaxis is an integral part for therapy. It should be prescribed from the moment of admission by IV administration of ceftriaxone at a dose of 1 g/d. Antibiotic prophylaxis should always be in accordance with local resistance patterns and antimicrobial policies. Antibiotic prophylaxis in LC patients with acute gastroesophageal variceal bleeding significantly reduces the frequency of bacterial infections, all-cause mortality, bacterial infection mortality, rebleeding events and hospitalization duration[41].
Malnutrition in LC patients with acute gastroesophageal variceal bleeding increases the risk of adverse outcomes, therefore, their feeding should be resumed 48-72 h after achieving hemostasis. Because of the lower cost and the lack of complications, enteral nutrition is always preferable to parenteral. If it is carried out through a nasogastric probe, manipulations with it should be performed with extreme caution should be performed with caution due to the risk of pulmonary infection[42].
Correction of hepatic encephalopathy in LC patients with acute gastroesophageal variceal bleeding is carried out by rapid removal of blood from the gastrointestinal tract by lactulose (through a nasogastric probe or in the form of enemas)[43].
Against the background of resuscitation measures in LC patients with acute upper gastrointestinal bleeding, EGDS should be performed within 12 h from the moment of admission. If the patient's condition is unstable, it is carried out as soon as possible, as far as it is safe. The diagnosis of gastroesophageal variceal bleeding is established by the presence of its active manifestations. In the absence of bleeding, indirect signs of the complication are the “white nipple sign” and blood clots on varices, as well as blood in the lumen of the esophagus and/or stomach if other possible causes have been ruled out[44]. Tracheal intubation before EGDS is recommended in patients with impaired consciousness and/or active vomiting blood. They are extubated immediately after the procedure is completed. In the absence of contraindications (QT interval prolongation), administration of intravenous erythromycin at 250 mg for 30-120 min before EGDS to improve mucosa visualization by enhancing gastric motility is considered appropriate[45].
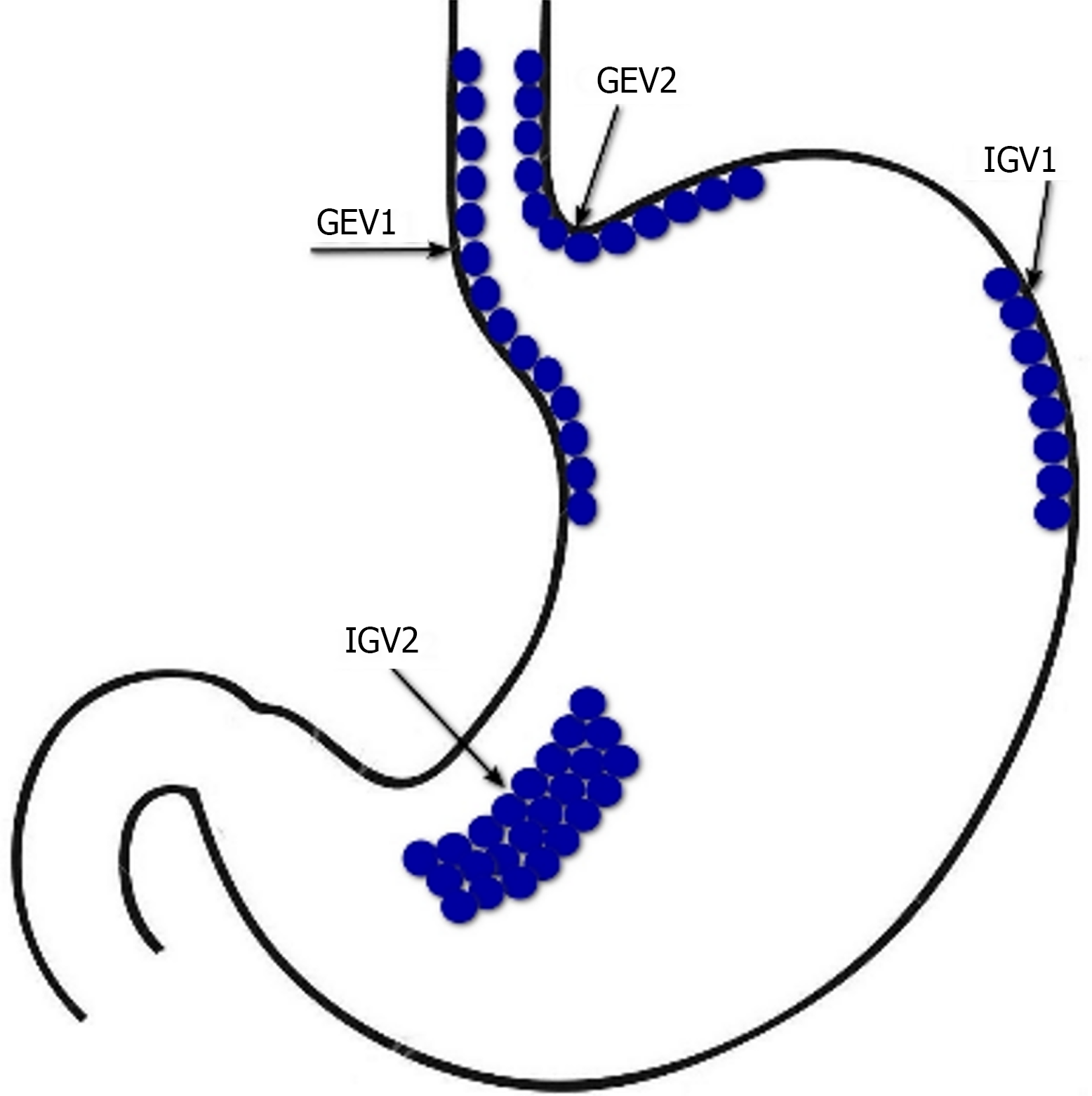
In LC patients with acute gastroesophageal variceal bleeding, the method of choice is EBL[5]. An additional 5-d course of pharmacotherapy with vasoactive drugs can significantly reduce the risk of their recurrence[46]. Endoscopic therapy with tissue adhesives (e.g. histoacryl (N-butyl-2-cyanoacrylate)/thrombin) is recommended for acute bleeding from type 1 GEV (GEV1) and type 2 GEV (GEV2) that extend beyond the cardia, and from type 1 and type 2 isolated gastric varices (IGV1 and IGV2) (Figure 2)[47]. In case of refractory gastroesophageal variceal bleeding despite combined pharmacotherapy with vasoactive drugs and EBL, esophageal balloon tamponade with a Sengstaken–Blakemore tube or the installation of a dedicated self-expandable, covered esophageal metal stent should be resorted to. Ideally, this should serve as a bridge to rescue PTFE-covered TIPS[48]. PTFE-covered TIPS is recommended in LC patients with uncontrolled acute gastroesophageal variceal bleeding at EGDS or who have successfully undergone EBL but who rebleed at any time during admission (after endoscopy). In addition, select LC patients Child-Turcotte-Pugh (CTP) class B or C with active gastroesophageal variceal bleeding at EGDS are at highest risk for rebleeding and may benefit from early or pre-emptive PTFE-covered TIPS within 72 h of admission to improve survival[49]. It has been shown that among selected advanced LC patients (CTP class B or C) with acute gastroesophageal variceal bleeding, PTFE-covered TIPS is superior to pharmacotherapy with vasoactive drugs plus EBL in improving transplantation-free survival, reducing failure to control bleeding, without increasing the risk of overt hepatic encephalopathy[50]. At the same time, TIPS may be useless in LC patients CTP class C with > 14 points, or with a MELD score > 30 and a lactate level > 12 mmol/L, if liver transplantation is not planned in the short term[5]. In patients with bleeding from GEV2 and from IGV1 and IGV2 balloon-occluded retrograde transvenous obliteration (BRTO) is possible as an alternative to endoscopic treatment or TIPS, provided that this is feasible (type and diameter of gastrorenal shunts) and there is experience in its use[51]. The combined use of TIPS and BRTO is possible both to control acute gastric variceal bleeding and to reduce the risk of their recurrence, particularly in cases when, despite a reduction in HVPG, portal flow remains diverted to gastrorenal shunts[47].
Since the cause of gastroesophageal variceal bleeding is PH, it is obvious that the basis of their treatment should be a reduction in portal pressure, and not correction of blood clotting disorders. Moreover, conventional coagulation screening parameters, for example, prothrombin time/international normalized ratio and activated partial thromboplastin time, reflect the hemostasis state in LC patients is not quite correct[52]. Therefore, fresh frozen plasma transfusion is not recommended in gastroesophageal variceal bleeding, since it will not correct coagulopathy and may lead to volume overload and worsening of PH[53]. This postulate was, in particular, confirmed in a multicentre cohort study, where fresh frozen plasma transfusion in acute gastroesophageal variceal bleeding was independently associated with poor clinical outcomes[54]. There is also no evidence that platelet count and fibrinogen levels are correlated with the risk of failure to control gastroesophageal variceal bleeding or rebleeding. In addition, the use of recombinant factor VIIa and tranexamic acid are not recommended in gastroesophageal variceal bleeding. At the same time, if pharmacotherapy with vasoactive drugs and/or EBL is ineffective, the decision to eliminate blood clotting disorders should be considered individually[53].
PH is the most important event of the natural history of LC, since it can be associated with its first and further decompensation and is responsible for the development of severe, often fatal complications, such as gastroesophageal variceal bleeding. The dynamic character and potential reversibility of LC requires the improvement of invasive and non-invasive methods of its diagnosis, as well as the identification of CSPH. This will allow to objectively and accurately stratify the risk of gastroesophageal variceal bleeding, develop individual strategies for their prevention and management, avoid the first and further decompensation in LC, which will improve the prognosis and survival of patients suffering from it (Figure 3).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey; Fan X, China S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
| 1. | D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 361] [Article Influence: 51.6] [Reference Citation Analysis (1)] |
| 2. | Jalan R, D'Amico G, Trebicka J, Moreau R, Angeli P, Arroyo V. New clinical and pathophysiological perspectives defining the trajectory of cirrhosis. J Hepatol. 2021;75 Suppl 1:S14-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | D'Amico G, Bernardi M, Angeli P. Towards a new definition of decompensated cirrhosis. J Hepatol. 2022;76:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (1)] |
| 4. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2268] [Article Influence: 226.8] [Reference Citation Analysis (3)] |
| 5. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1394] [Article Influence: 464.7] [Reference Citation Analysis (2)] |
| 6. | Bosch J, Garcia-Pagán JC, Berzigotti A, Abraldes JG. Measurement of portal pressure and its role in the management of chronic liver disease. Semin Liver Dis. 2006;26:348-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Karagiannakis DS, Voulgaris T, Siakavellas SI, Papatheodoridis GV, Vlachogiannakos J. Evaluation of portal hypertension in the cirrhotic patient: hepatic vein pressure gradient and beyond. Scand J Gastroenterol. 2018;53:1153-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute Technical Review on the Role of Elastography in Chronic Liver Diseases. Gastroenterology. 2017;152:1544-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 209] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 9. | European Association for the Study of the Liver. Clinical Practice Guideline Panel; Chair:; EASL Governing Board representative:; Panel members:. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1009] [Article Influence: 252.3] [Reference Citation Analysis (0)] |
| 10. | Lee E, Kim YJ, Goo DE, Yang SB, Kim HJ, Jang JY, Jeong SW. Comparison of hepatic venous pressure gradient and endoscopic grading of esophageal varices. World J Gastroenterol. 2016;22:3212-3219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Garbuzenko DV, Arefyev NO. Primary prevention of bleeding from esophageal varices in patients with liver cirrhosis: An update and review of the literature. J Evid Based Med. 2020;13:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Hofer BS, Simbrunner B, Hartl L, Jachs M, Bauer DJM, Balcar L, Paternostro R, Schwabl P, Semmler G, Scheiner B, Staettermayer AF, Trauner M, Mandorfer M, Reiberger T. Alcohol Abstinence Improves Prognosis Across All Stages of Portal Hypertension in Alcohol-Related Cirrhosis. Clin Gastroenterol Hepatol. 2023;21:2308-2317.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 54] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 13. | Garbuzenko DV. [The role of antiviral therapy in the management of patients with liver cirrhosis associated with chronic HBV and HCV infection]. Vopr Virusol. 2021;66:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Gratacós-Ginès J, Pose E. Review of the role of statins in cirrhosis and portal hypertension. Clin Liver Dis (Hoboken). 2023;22:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Turco L, Schepis F, Villa E. The Role of Anticoagulation in Treating Portal Hypertension. Curr Hepatol Rep. 2018;17:200-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Garbuzenko DV. Therapeutic possibilities of gut microbiota modulation in acute decompensation of liver cirrhosis. World J Hepatol. 2023;15:525-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 17. | Garbuzenko DV. Current strategies for targeted therapy of liver fibrosis. Bulletin of Siberian Medicine. 2022;21:154-165. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 18. | Garbuzenko DV. Contemporary concepts of the medical therapy of portal hypertension under liver cirrhosis. World J Gastroenterol. 2015;21:6117-6126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 19. | Wong YJ, Zhaojin C, Tosetti G, Degasperi E, Sharma S, Agarwal S, Chuan L, Huak CY, Jia L, Xiaolong Q, Saraya A, Primignani M. Baveno-VII criteria to predict decompensation and initiate non-selective beta-blocker in compensated advanced chronic liver disease patients. Clin Mol Hepatol. 2023;29:135-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 20. | Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, Bañares R, Morillas RM, Poca M, Peñas B, Augustin S, Abraldes JG, Alvarado E, Torres F, Bosch J. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 432] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 21. | Rowe IA, Villanueva C, Shearer JE, Torres F, Albillos A, Genescà J, Garcia-Pagan JC, Tripathi D, Hayes PC, Bosch J, Abraldes JG; PREDESCI trial investigators. Quantifying the benefit of nonselective beta-blockers in the prevention of hepatic decompensation: A Bayesian reanalysis of the PREDESCI trial. Hepatology. 2023;78:530-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Turco L, Villanueva C, La Mura V, García-Pagán JC, Reiberger T, Genescà J, Groszmann RJ, Sharma BC, Merkel C, Bureau C, Alvarado E, Abraldes JG, Albillos A, Bañares R, Peck-Radosavljevic M, Augustin S, Sarin SK, Bosch J, García-Tsao G. Lowering Portal Pressure Improves Outcomes of Patients With Cirrhosis, With or Without Ascites: A Meta-Analysis. Clin Gastroenterol Hepatol. 2020;18:313-327.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 23. | Garbuzenko DV. [Aspects of pathogenetc pharmacotherapy for portal hypertension in liver cirrhosis]. Ter Arkh. 2016;88:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Ivashkin VT, Maevskaya MV, Zharkova MS, Zhigalova SB, Kitsenko EA, Manukyan GV, Trukhmanov AS, Maev IV, Tikhonov IN, Deeva TA. Clinical Recommendations of the Russian Scientific Liver Society and Russian Gastroenterological Association on Diagnosis and Treatment of Liver Fibrosis, Cirrhosis and Their Complications. Rus J Gastroenterol Hepatol Coloproctol. 2021;31:56-102. |
| 25. | Sinagra E, Perricone G, D'Amico M, Tinè F, D'Amico G. Systematic review with meta-analysis: the haemodynamic effects of carvedilol compared with propranolol for portal hypertension in cirrhosis. Aliment Pharmacol Ther. 2014;39:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Dardari L, Taha M, Dahat P, Toriola S, Satnarine T, Zohara Z, Adelekun A, Seffah KD, Salib K, Arcia Franchini AP. The Efficacy of Carvedilol in Comparison to Propranolol in Reducing the Hepatic Venous Pressure Gradient and Decreasing the Risk of Variceal Bleeding in Adult Cirrhotic Patients: A Systematic Review. Cureus. 2023;15:e43253. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Kirnake V, Arora A, Gupta V, Sharma P, Singla V, Bansal N, Goyal M, Chawlani R, Kumar A. Hemodynamic Response to Carvedilol is Maintained for Long Periods and Leads to Better Clinical Outcome in Cirrhosis: A Prospective Study. J Clin Exp Hepatol. 2016;6:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1775] [Article Influence: 253.6] [Reference Citation Analysis (2)] |
| 29. | Thiele M, Krag A, Rohde U, Gluud LL. Meta-analysis: banding ligation and medical interventions for the prevention of rebleeding from oesophageal varices. Aliment Pharmacol Ther. 2012;35:1155-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Pfisterer N, Dexheimer C, Fuchs EM, Bucsics T, Schwabl P, Mandorfer M, Gessl I, Sandrieser L, Baumann L, Riedl F, Scheiner B, Pachofszky T, Dolak W, Schrutka-Kölbl C, Ferlitsch A, Schöniger-Hekele M, Peck-Radosavljevic M, Trauner M, Madl C, Reiberger T. Betablockers do not increase efficacy of band ligation in primary prophylaxis but they improve survival in secondary prophylaxis of variceal bleeding. Aliment Pharmacol Ther. 2018;47:966-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Jachs M, Hartl L, Simbrunner B, Bauer D, Paternostro R, Balcar L, Hofer B, Pfisterer N, Schwarz M, Scheiner B, Stättermayer AF, Pinter M, Trauner M, Mandorfer M, Reiberger T. Carvedilol Achieves Higher Hemodynamic Response and Lower Rebleeding Rates Than Propranolol in Secondary Prophylaxis. Clin Gastroenterol Hepatol. 2023;21:2318-2326.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 32. | Reiberger T, Mandorfer M. Beta adrenergic blockade and decompensated cirrhosis. J Hepatol. 2017;66:849-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 33. | Tripathi D, Stanley AJ, Hayes PC, Travis S, Armstrong MJ, Tsochatzis EA, Rowe IA, Roslund N, Ireland H, Lomax M, Leithead JA, Mehrzad H, Aspinall RJ, McDonagh J, Patch D. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69:1173-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 212] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 34. | Qi X, Tian Y, Zhang W, Zhao H, Han G, Guo X. Covered TIPS for secondary prophylaxis of variceal bleeding in liver cirrhosis: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95:e5680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Larrue H, D'Amico G, Olivas P, Lv Y, Bucsics T, Rudler M, Sauerbruch T, Hernandez-Gea V, Han G, Reiberger T, Thabut D, Vinel JP, Péron JM, García-Pagán JC, Bureau C. TIPS prevents further decompensation and improves survival in patients with cirrhosis and portal hypertension in an individual patient data meta-analysis. J Hepatol. 2023;79:692-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 49] [Article Influence: 24.5] [Reference Citation Analysis (1)] |
| 36. | Gao L, Li MB, Li JY, Liu Y, Ren C, Feng DP. Impressive recompensation in transjugular intrahepatic portosystemic shunt-treated individuals with complications of decompensated cirrhosis based on Baveno VII criteria. World J Gastroenterol. 2023;29:5383-5394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (3)] |
| 37. | Garbuzenko DV. Current approaches to the management of patients with liver cirrhosis who have acute esophageal variceal bleeding. Curr Med Res Opin. 2016;32:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Quraishi MN, Khan F, Tripathi D. How we manage variceal hemorrhage in cirrhotic patients. Key practical messages from the British Guidelines. Pol Arch Med Wewn. 2016;126:174-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Huaringa-Marcelo J, Huaman MR, Brañez-Condorena A, Villacorta-Landeo P, Pinto-Ruiz DF, Urday-Ipanaqué D, García-Gomero D, Montes-Teves P, Lozano Miranda A. Vasoactive Agents for the Management of Acute Variceal Bleeding: A Systematic Review and Meta-analysis. J Gastrointestin Liver Dis. 2021;30:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Hwang SJ, Lee DH, Koh SJ, Kim JW, Park HS, Kim BG, Lee KL. Correlation Between Proton Pump Inhibitors and the Complications of Liver Cirrhosis: A Systematic Review and Meta-Analysis. Turk J Gastroenterol. 2022;33:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, Soares-Weiser K, Mendez-Sanchez N, Gluud C, Uribe M. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding - an updated Cochrane review. Aliment Pharmacol Ther. 2011;34:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 42. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 654] [Article Influence: 109.0] [Reference Citation Analysis (2)] |
| 43. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatic encephalopathy. J Hepatol. 2022;77:807-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 208] [Article Influence: 69.3] [Reference Citation Analysis (1)] |
| 44. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1417] [Article Influence: 177.1] [Reference Citation Analysis (3)] |
| 45. | Orpen-Palmer J, Stanley AJ. Update on the management of upper gastrointestinal bleeding. BMJ Med. 2022;1:e000202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 46. | Yan P, Tian X, Li J. Is additional 5-day vasoactive drug therapy necessary for acute variceal bleeding after successful endoscopic hemostasis?: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e12826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Henry Z, Patel K, Patton H, Saad W. AGA Clinical Practice Update on Management of Bleeding Gastric Varices: Expert Review. Clin Gastroenterol Hepatol. 2021;19:1098-1107.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 48. | Escorsell À, Pavel O, Cárdenas A, Morillas R, Llop E, Villanueva C, Garcia-Pagán JC, Bosch J; Variceal Bleeding Study Group. Esophageal balloon tamponade versus esophageal stent in controlling acute refractory variceal bleeding: A multicenter randomized, controlled trial. Hepatology. 2016;63:1957-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 49. | Boike JR, Thornburg BG, Asrani SK, Fallon MB, Fortune BE, Izzy MJ, Verna EC, Abraldes JG, Allegretti AS, Bajaj JS, Biggins SW, Darcy MD, Farr MA, Farsad K, Garcia-Tsao G, Hall SA, Jadlowiec CC, Krowka MJ, Laberge J, Lee EW, Mulligan DC, Nadim MK, Northup PG, Salem R, Shatzel JJ, Shaw CJ, Simonetto DA, Susman J, Kolli KP, VanWagner LB; Advancing Liver Therapeutic Approaches (ALTA) Consortium. North American Practice-Based Recommendations for Transjugular Intrahepatic Portosystemic Shunts in Portal Hypertension. Clin Gastroenterol Hepatol. 2022;20:1636-1662.e36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 50. | Lv Y, Yang Z, Liu L, Li K, He C, Wang Z, Bai W, Guo W, Yu T, Yuan X, Zhang H, Xie H, Yao L, Wang J, Li T, Wang Q, Chen H, Wang E, Xia D, Luo B, Li X, Yuan J, Han N, Zhu Y, Niu J, Cai H, Xia J, Yin Z, Wu K, Fan D, Han G; AVB-TIPS Study Group. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:587-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 51. | Gimm G, Chang Y, Kim HC, Shin A, Cho EJ, Lee JH, Yu SJ, Yoon JH, Kim YJ. Balloon-Occluded Retrograde Transvenous Obliteration versus Transjugular Intrahepatic Portosystemic Shunt for the Management of Gastric Variceal Bleeding. Gut Liver. 2018;12:704-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | O'Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA Clinical Practice Update: Coagulation in Cirrhosis. Gastroenterology. 2019;157:34-43.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 280] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 53. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on prevention and management of bleeding and thrombosis in patients with cirrhosis. J Hepatol. 2022;76:1151-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 182] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 54. | Mohanty A, Kapuria D, Canakis A, Lin H, Amat MJ, Rangel Paniz G, Placone NT, Thomasson R, Roy H, Chak E, Baffy G, Curry MP, Laine L, Rustagi T. Fresh frozen plasma transfusion in acute variceal haemorrhage: Results from a multicentre cohort study. Liver Int. 2021;41:1901-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |